Tracing the Origins of Darifenacin
When we talk about the history of Darifenacin, we are venturing into the realm of medical advancements in the 20th century. Darifenacin, a product of the pharmaceutical industry, was developed to help those who suffer from an overactive bladder. The discovery of this drug is actually a fascinating story. It all started in the labs where scientists were trying to develop a solution that could alleviate the discomfort of overactive bladder syndrome. They ended up creating Darifenacin, a medication that has since been instrumental in improving the lives of countless individuals around the world.
Over the years, the formula has been tweaked and improved to offer better relief and fewer side effects. What we have today is a highly developed version of Darifenacin that is far superior to its initial form. But the story of how we arrived here is truly fascinating and worth delving into.
The Pharmacological Evolution of Darifenacin
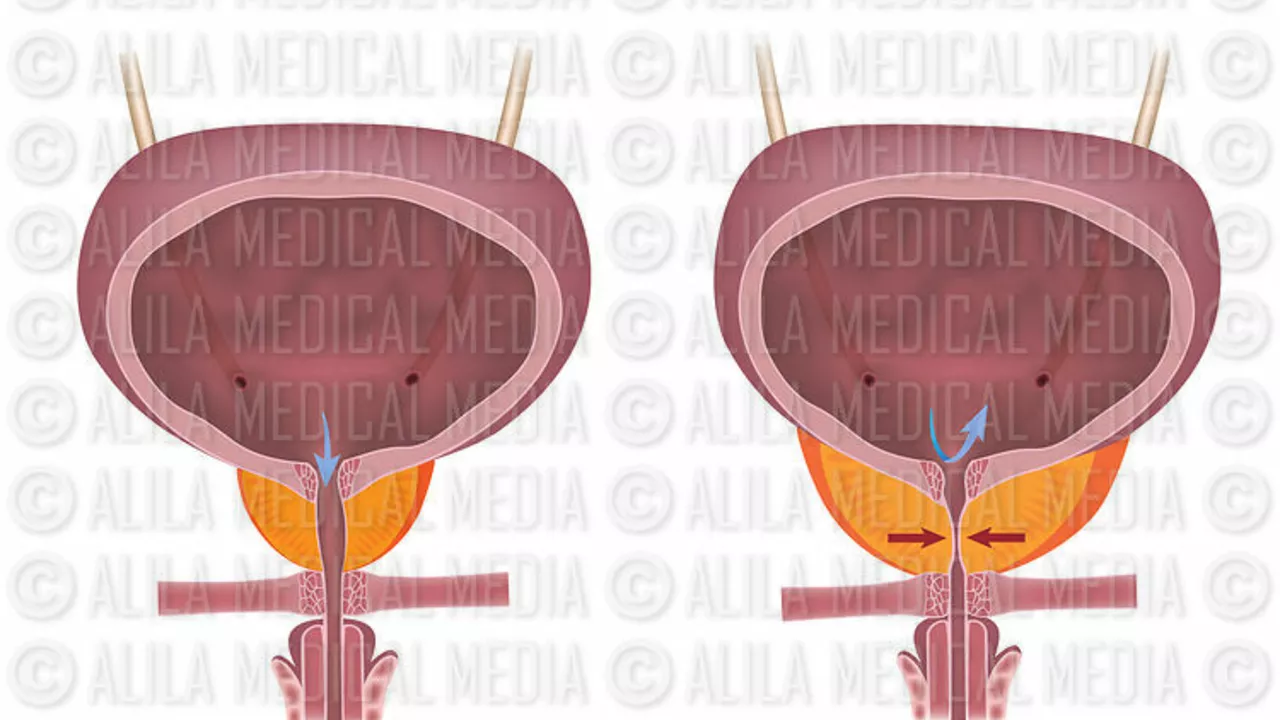
Darifenacin, as we know it today, is a product of years of research and pharmacological evolution. The drug was initially developed to specifically target the M3 receptors in the bladder, which are responsible for bladder contraction. The idea was to decrease the activity of these receptors, thereby reducing the symptoms of an overactive bladder.
Through rigorous testing and trials, the drug was refined and its effectiveness verified. This led to Darifenacin being approved for medical use in the United States in 2004. Over the years, further studies and advancements have allowed for the drug's formula to be refined, increasing its effectiveness and decreasing any potential side effects. It's a testament to the power of scientific progress and the relentless pursuit of better treatments for those in need.
Darifenacin and Its Impact on Patients
One cannot discuss the history and development of Darifenacin without mentioning its impact on patients. Since its introduction, Darifenacin has proven to be a game-changer for those suffering from an overactive bladder. The relief it offers has given patients the ability to lead a normal life, without the constant worry and discomfort that the condition brings.
The impact of Darifenacin extends beyond just physical relief. It has also contributed to the mental well-being of patients. Those who have an overactive bladder often suffer from anxiety and stress due to their condition. Darifenacin has helped to alleviate these psychological issues, allowing patients to lead happier and healthier lives.
The Ongoing Research and Future of Darifenacin
The story of Darifenacin doesn't end with its current use. As with any medication, ongoing research is being conducted to further improve its effectiveness and minimize its side effects. Scientists are constantly looking for ways to refine and improve the drug, ensuring that it continues to provide the best possible relief for patients.
Looking ahead, the future of Darifenacin is promising. With the continued advancements in medical technology and pharmacology, there is the potential for even more effective and safer versions of the drug. Only time will tell what the future holds, but one thing is clear: Darifenacin will continue to play an important role in treating an overactive bladder.
Darifenacin: A Medical Marvel
In conclusion, the history and development of Darifenacin is a testament to the power of scientific progress and the relentless pursuit of better treatments for those in need. It's a story of how a simple idea in a lab can lead to a medical marvel that improves the lives of countless individuals around the world. It's a story that reminds us of the importance of medical research and the impact it can have on our lives.
And so, as we trace the journey of Darifenacin from its humble beginnings to its current prominence, we are reminded of the power of human ingenuity and the potential of medical science. It's a story that is continuing to unfold, and one that is worth keeping an eye on.

Amy Collins
Honestly, the whole Darifenacin saga reads like a textbook on pharma buzzwords.
amanda luize
What a spectacularly glossy narrative, peppered with half‑baked claims and a suspiciously immaculate safety record – it's almost as if a secret cabal of marketing execs decided to dress up the mundane chemistry of M3 antagonism in a shimmering cloak of miracles. The language is drenched in jargon, yet the underlying message feels like a veiled advertisement for a profit‑driven agenda. One can't help but wonder who funds the endless stream of "refinements" that keep the price tags climbing. It reads like a covert recruitment pamphlet for the next wave of pharmaceutical zealots.
Chris Morgan
The drug was approved, not because it was flawless, but because the data was presented just so.
Pallavi G
Hey folks! Just wanted to add that Darifenacin works by selectively blocking M3 receptors in the bladder, which really helps reduce involuntary contractions. This mechanism means fewer trips to the bathroom and a noticeable boost in quality of life. If you're considering it, talk to your urologist about dosing – they can tailor it to your specific symptoms. And remember, stay hydrated but avoid excessive caffeine, as it can counteract the benefits.
Rafael Lopez
Indeed, the pharmacodynamics of Darifenacin are quite fascinating; by antagonizing the M3 subtype, it diminishes cholinergic stimulation, thereby reducing detrusor overactivity. Moreover, the drug’s half‑life allows for once‑daily dosing, which improves patient adherence, especially in the elderly population. Clinical trials have demonstrated statistically significant improvements in urgency episodes, and the side‑effect profile is relatively favorable compared to older antimuscarinics. It’s also worth noting that the formulation includes a controlled‑release matrix, which smooths plasma concentration peaks and troughs.
gary kennemer
Reflecting on the evolution of Darifenacin, one sees a microcosm of modern drug development: a relentless pursuit of efficacy tempered by the ethical imperative to minimize harm. The iterative refinements remind us that scientific progress is seldom linear; each modification builds upon the lessons of its predecessor, echoing the broader philosophical truth that knowledge is cumulative.
Payton Haynes
Everything looks clean on paper, but the hidden agenda is clear – pharma companies push drugs like Darifenacin to lock us into lifelong medication cycles. The data is selectively reported, and the long‑term effects are still a mystery. Stay skeptical and demand full transparency.
Earlene Kalman
Honestly, the article glosses over the side‑effects; patients report dry mouth, constipation, and sometimes blurred vision, yet the write‑up feels like a PR piece.
Brian Skehan
Another day, another “miracle” pill – looks like they’re just repackaging the same old chemistry with a fancier name.
Andrew J. Zak
While it’s easy to jump on the criticism bandwagon, it’s also important to recognize the genuine relief Darifenacin has offered to many patients across cultures. The collaborative efforts between researchers worldwide have paved the way for a medication that, despite its imperfections, addresses a real need in the global health landscape.
Dominique Watson
From a British perspective, the United States’ reliance on proprietary drugs like Darifenacin underscores a troubling over‑dependence on corporate interests rather than public‑owned research.
Mia Michaelsen
Fact check: Darifenacin received FDA approval in 2004 after phase III trials demonstrated a 25% reduction in urgency episodes versus placebo. The drug’s metabolism is primarily hepatic via CYP3A4, so clinicians must watch for interactions with strong inhibitors like ketoconazole. Its once‑daily dosing stems from a 13‑hour half‑life, providing steady plasma levels. The most common adverse events reported were dry mouth (≈ 31%) and constipation (≈ 15%).
AARON KEYS
Just a quick note: the term “pharmacological evolution” is fine, but “refined” should be followed by a comma when used in a non‑restrictive clause. Minor nitpick, but clarity matters.
Melissa Shore
The trajectory of Darifenacin, from its initial discovery phase in the late 1990s to its eventual market introduction in the early 2000s, illustrates a quintessential example of iterative drug development, wherein each successive trial not only validated the compound’s efficacy but also illuminated nuances in its safety profile that would later inform dosage adjustments, formulation enhancements, and patient selection criteria; the Phase I studies, conducted primarily on healthy volunteers, established a baseline tolerability threshold, which, when juxtaposed with the more expansive Phase II and III cohorts comprising patients with overactive bladder, revealed a statistically significant reduction in urgency episodes, thereby cementing the drug’s therapeutic promise, and it was this compelling evidence that ultimately persuaded regulatory bodies to grant approval in 2004, a milestone that nonetheless marked only the commencement of a broader post‑marketing surveillance effort aimed at capturing real‑world outcomes; subsequent pharmacovigilance data underscored the prevalence of anticholinergic side effects such as xerostomia and constipation, prompting formulators to introduce a controlled‑release tablet designed to mitigate peak plasma concentrations, and further research endeavored to delineate drug–drug interactions, particularly with agents metabolized via CYP3A4, leading to updated prescribing information that emphasized caution when co‑administered with potent inhibitors; as the scientific community continues to dissect the mechanistic pathways underlying bladder contractility, newer generations of M3 antagonists have emerged, each striving to balance efficacy with tolerability, yet Darifenacin remains a benchmark against which these novel agents are measured, reflecting both its historical significance and its enduring clinical relevance in the therapeutic armamentarium for overactive bladder.
Michelle Pellin
The saga of Darifenacin reads like a dramatic opera – from the humble laboratory whispers to the thunderous applause of FDA approval, each act brims with tension, triumph, and the lingering scent of ambition.
Keiber Marquez
Look, if we keep pushing American drugs worldwide we’ll just keep paying more and more.
Lily Saeli
Morally speaking, we should question whether profiting from a condition that causes personal shame is ever truly ethical.